In a paper printed past thirty day period in Nano Letters, the staff explain how they’ve designed a novel “fireproof” strong-condition electrolyte (SSE) for use in lithium-ion batteries. “We tackle the dilemma of flammability in SSEs by including a fire retardant,” suggests Jiayu Wan, a postdoctoral researcher in Cui’s lab and co-author of the paper.
They used a flame-retardant materials known as decabromodiphenyl ethane, or DBDPE for small. To make their new strong-condition electrolyte, the staff 1st designed a slim film by combining DBDPE with polyimide, a mechanical enforcer.
Making use of polyimide has several positive aspects, suggests Wan. Aside from currently being “mechanically actually robust,” it features a higher melting level (making it fewer probable that a small circuit will arise), a options-dependent production approach (that is appropriate with how batteries are created currently), and it is affordable (3M even has film tapes created from it).
The hitch, nevertheless, is that polyimide can’t perform ions. To get all over this snag, Wan and his colleagues added two different polymers, polyethylene oxide (PEO) and lithium bistrifluoromethanesulfonylimide (LiTFSI), to the blend.
“It’s innovative—they’ve well used co-polymers, which is a new way to remedy the flammable polymer electrolyte battery dilemma,” suggests Chunsheng Wang, a researcher who research new battery technologies at the College of Maryland.
Good-condition electrolytes acquire two key sorts. You can make them from ceramics, a materials that conducts ions nicely but is very brittle and results in thick batteries, which have lessen vitality density. Or, you can have electrolytes composed of polymers, which are lower charge, lightweight, and flexible. They’re also “soft,” which means there is lower resistance alongside the interface of the electrode and electrolyte, which makes it possible for the electrolyte to perform ions simply.
But polymer electrolytes also have difficulties. “This softness implies they’re not able to suppress lithium dendrite propagation, so they’re flammable,” suggests Wang, referring to the small needle-like projections that increase from a battery’s anode. Dendrites can final result following repeated cycles of charging and discharging when these lithium crystals pierce a battery’s separator, they can start off fires.
“A good deal of men and women think that for liquid electrolytes, there is no resistance and dendrites can increase by way of the electrolyte,” suggests Wang. “But if you substitute the liquid with a strong, which is mechanically more powerful, the lithium might be blocked.”
Their mechanical strength, alongside with lowered flammability, are just some causes why strong-condition electrolytes have garnered interest among the researchers in each academia and industry. A 3rd motive lies with the truth that they make it possible for batteries to be stacked. “Because the electrolyte does not flow, you can simply place them jointly without having wires… which is significant for rising vitality density,” suggests Wang.
There’s no ideal option, nevertheless. “All the different SSEs have some difficulties, so you have to balance them out,” he suggests.
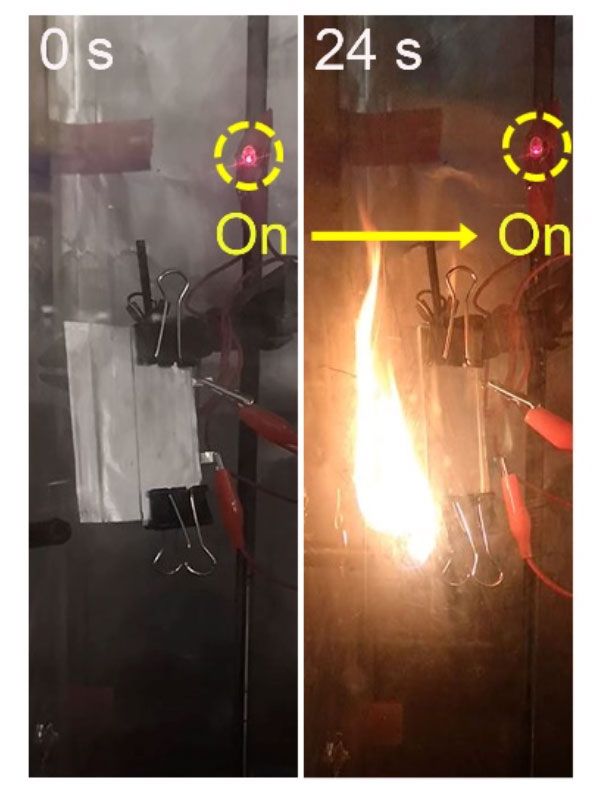
It is a aim that the staff at Stanford seems one particular phase nearer to achieving. Not only is their new strong-condition electrolyte ultrathin (measuring in between ten to 25 micrometers), it also provides a higher unique ability (131 milliampere several hours for every gram, mAh/g, at one diploma C), and demonstrates good cycling functionality (lasting 300 cycles at sixty levels C). Crucially, prototype battery cells created making use of it proved to work inspite of catching fire (in this video clip, an LED stays lit even nevertheless the battery powering it is on fire).
“This was very shocking to us,” suggests Stanford’s Wan. “Usually a battery will just explode with a fire. But with this one particular, not only does it not explode, it continue to functions.”
These days, the staff proceeds to discover new components and buildings for use in strong-condition electrolytes, with the aim of boosting existing density and cell ability. Suggests Wan: “The obstacle now is to make the battery demand a lot quicker, have a greater vitality density, and to past extended.”
